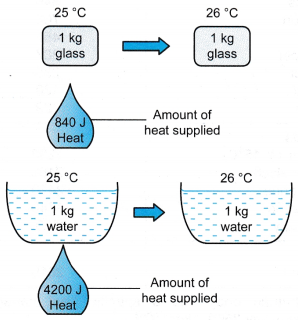
Breakdown of One-to-One Correspondence in Energy and Volume in a High-Pressure Heat-Treated Zr-Based Metallic Glass During Annealing | Scientific Reports
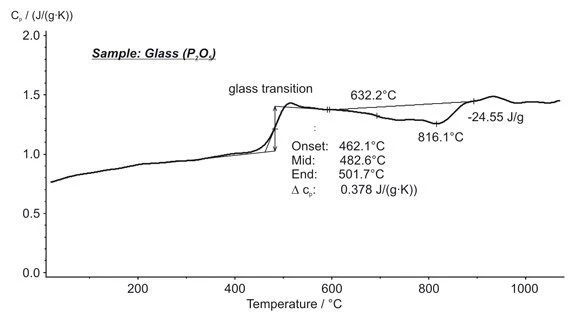
Phosphate Glass Powder — Glass Transition, Structural Change, Specific Heat - NETZSCH Analyzing & Testing

Specific heat and magnetization studies of spin-glass like transition in nanogranular Cu90Co10 ribbon - ScienceDirect

Heat capacity (C p ) vs. temperature curves in the glass transition... | Download Scientific Diagram
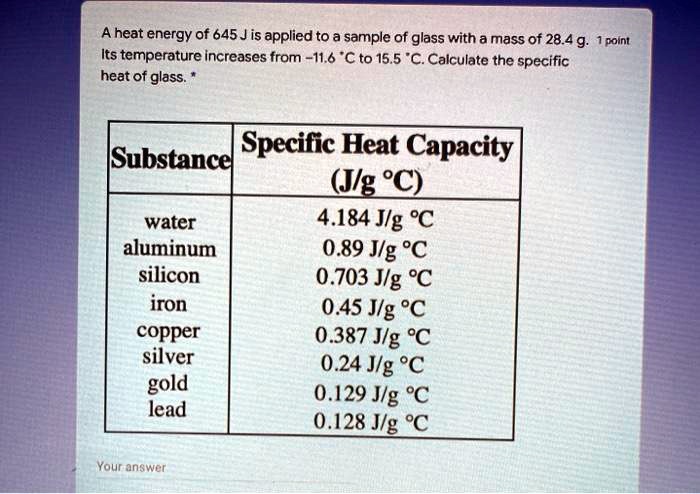
SOLVED: Aheat energy of 645 J is applied to a sample of glass with a mass of 28.4g: point Its temperature increases from -11.6 'C to 15.5 'C. Calculate the specific heat
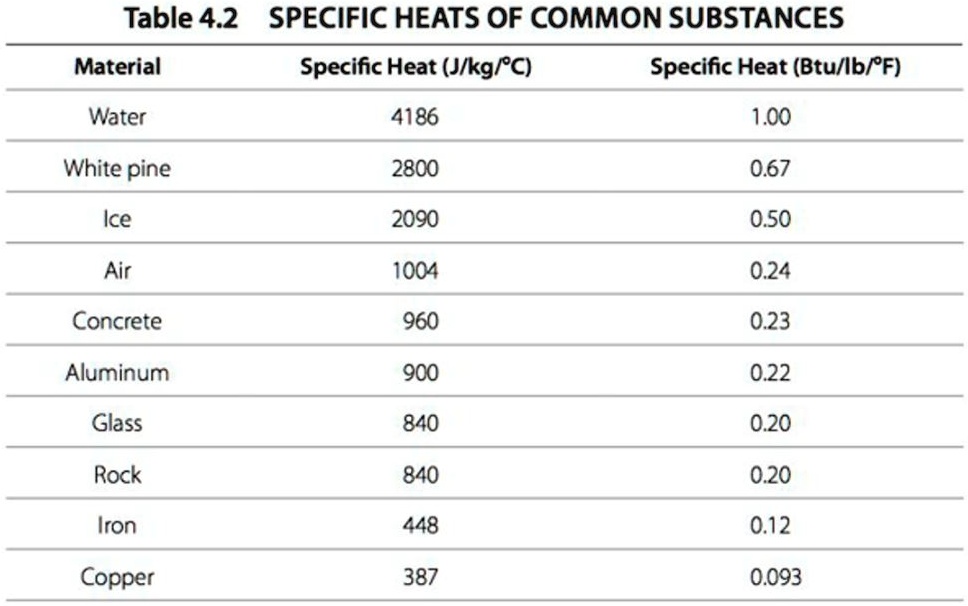
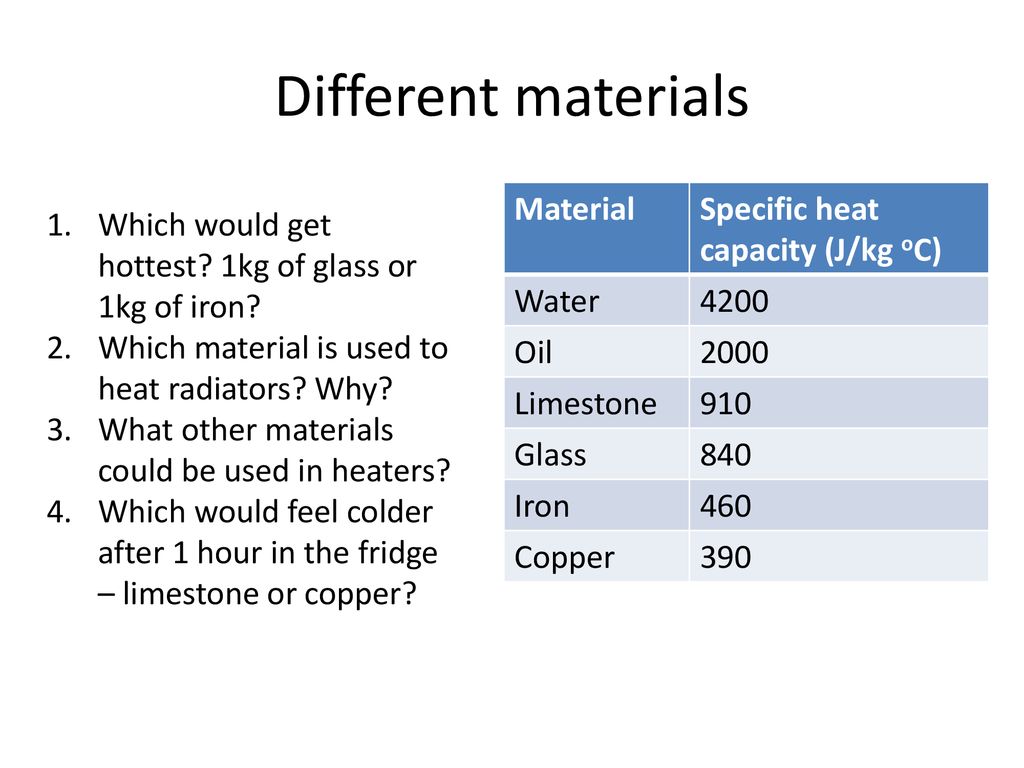
SOLVED: Table 4.2 SPECIFIC HEATS OF COMMON SUBSTANCES Material Specific Heat (JlkgrC) Specific Heat (BtullbrF) Water 4186 1.00 White pine 2800 0.67 Ice 2090 0.50 Air 1004 0.24 Concrete 960 0.23 Aluminum 900 0.22 Glass 840 0.20 Rock 840 0.20 Iron 448 ...



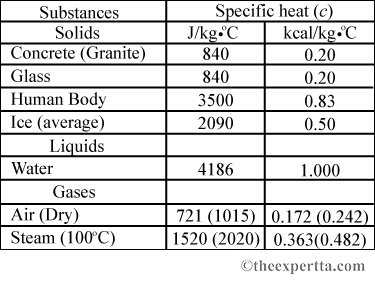
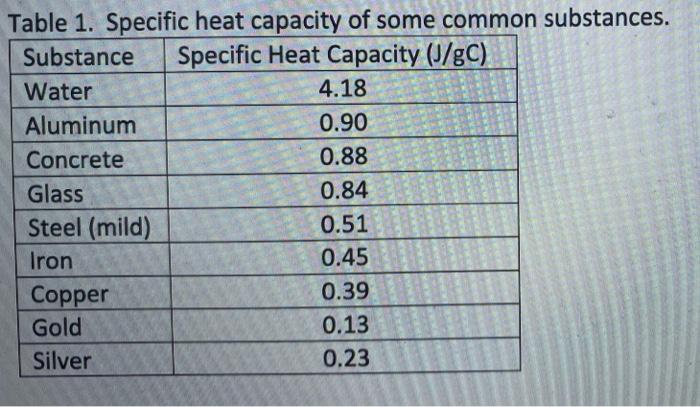
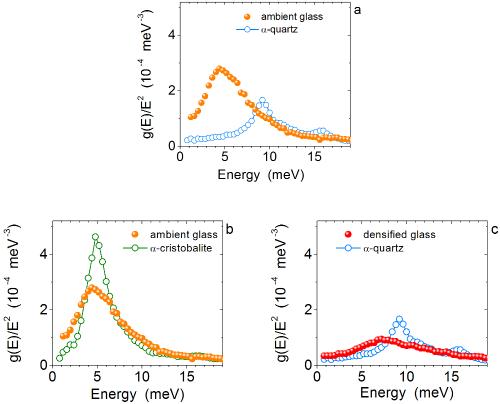
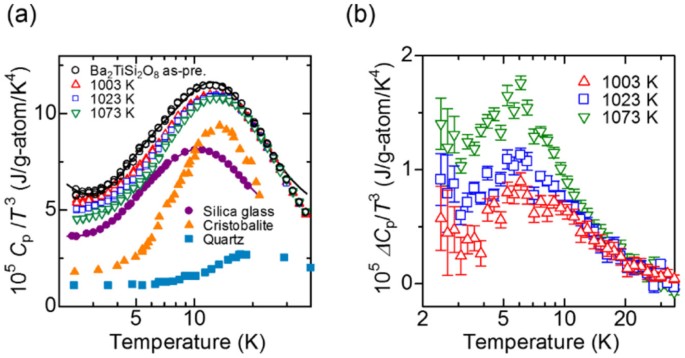



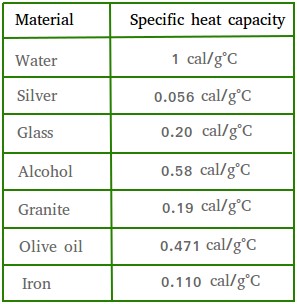
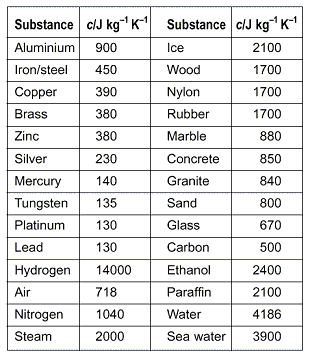
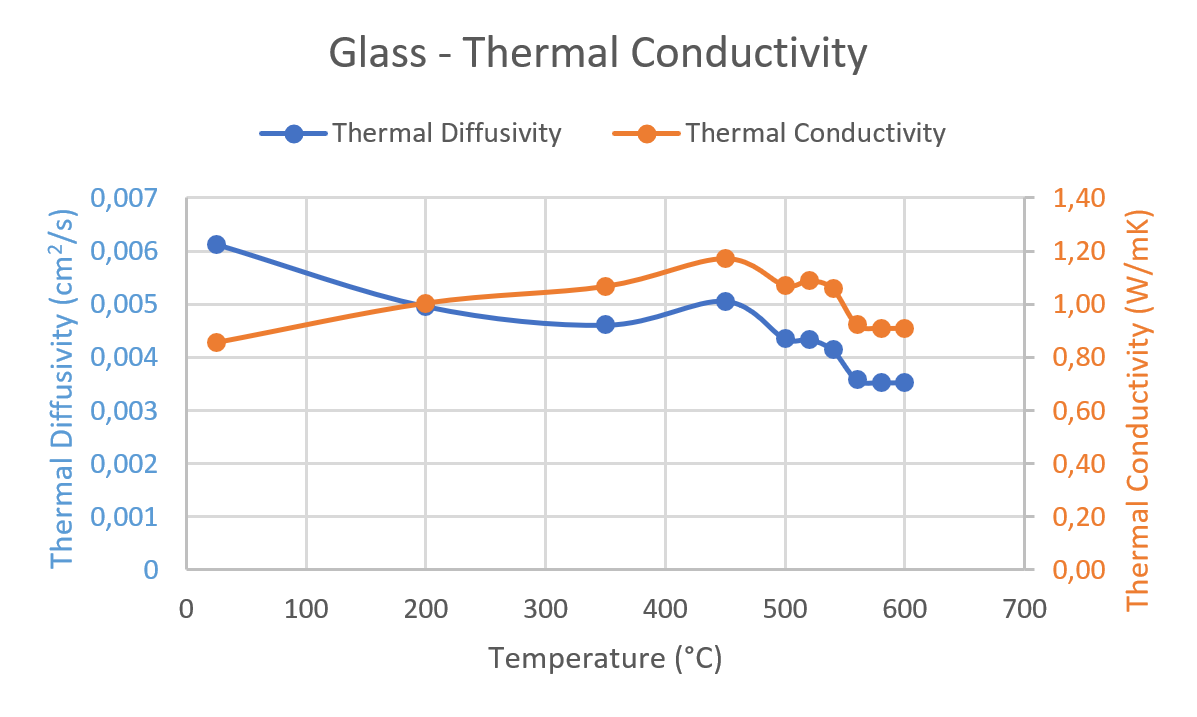
.jpg)