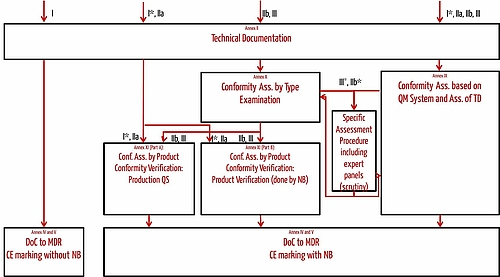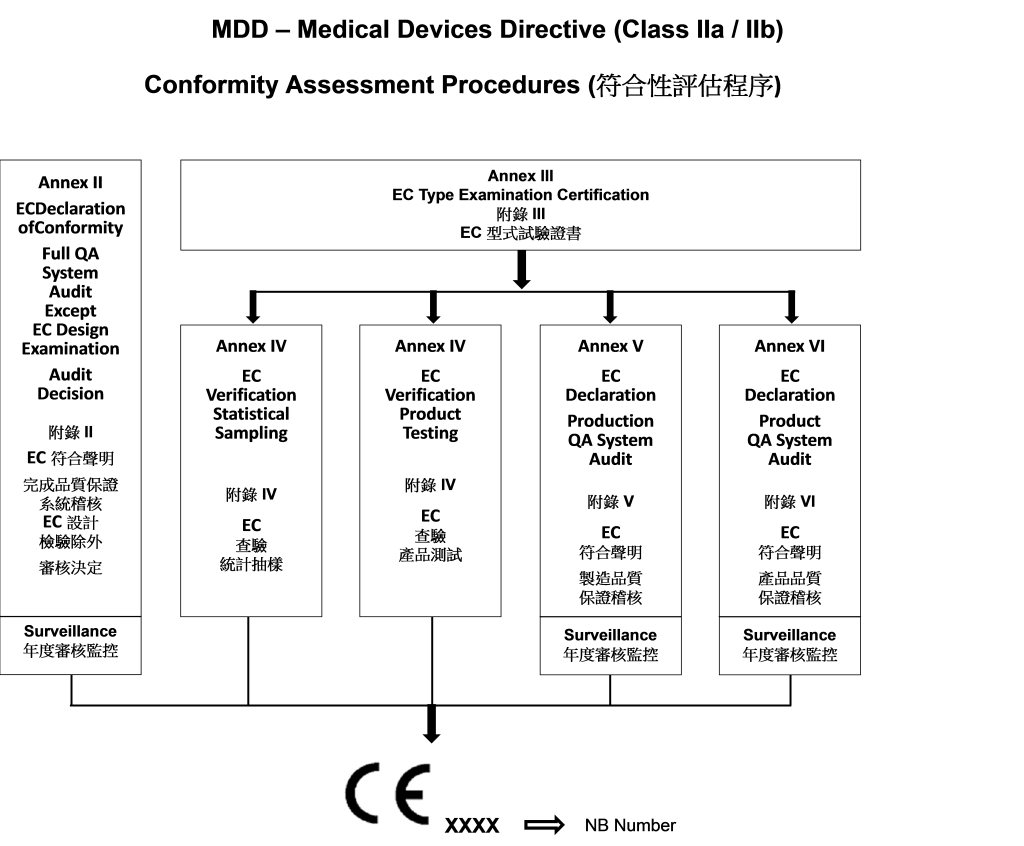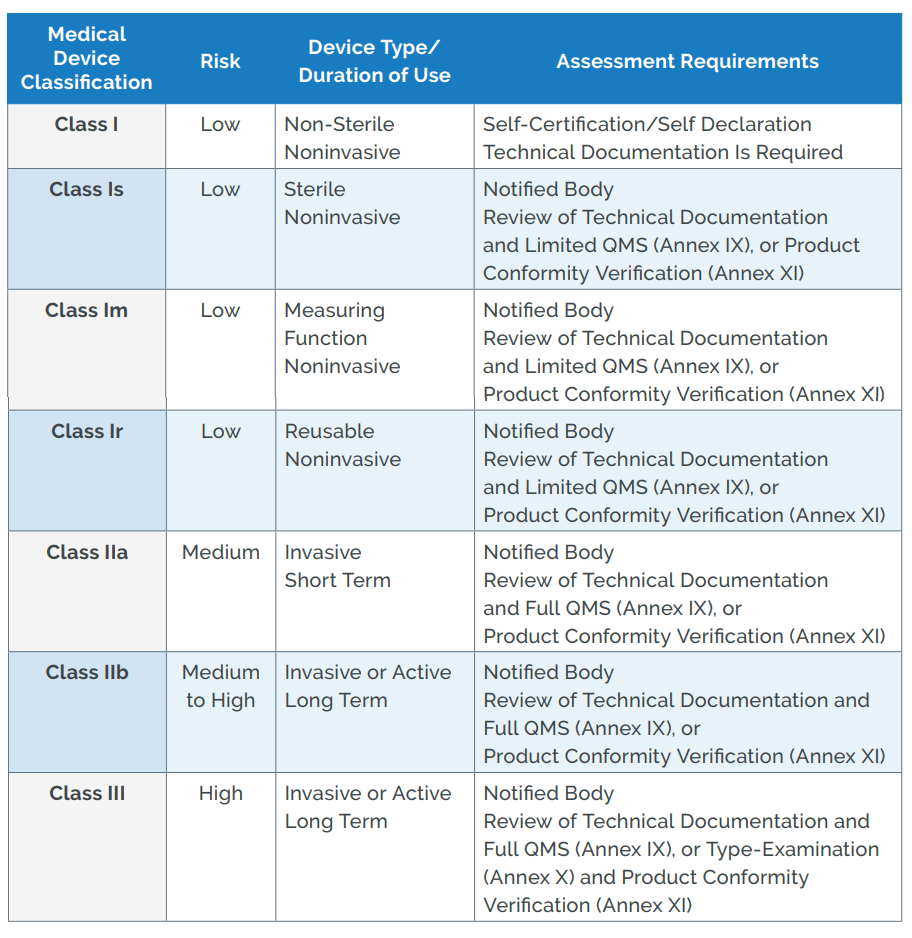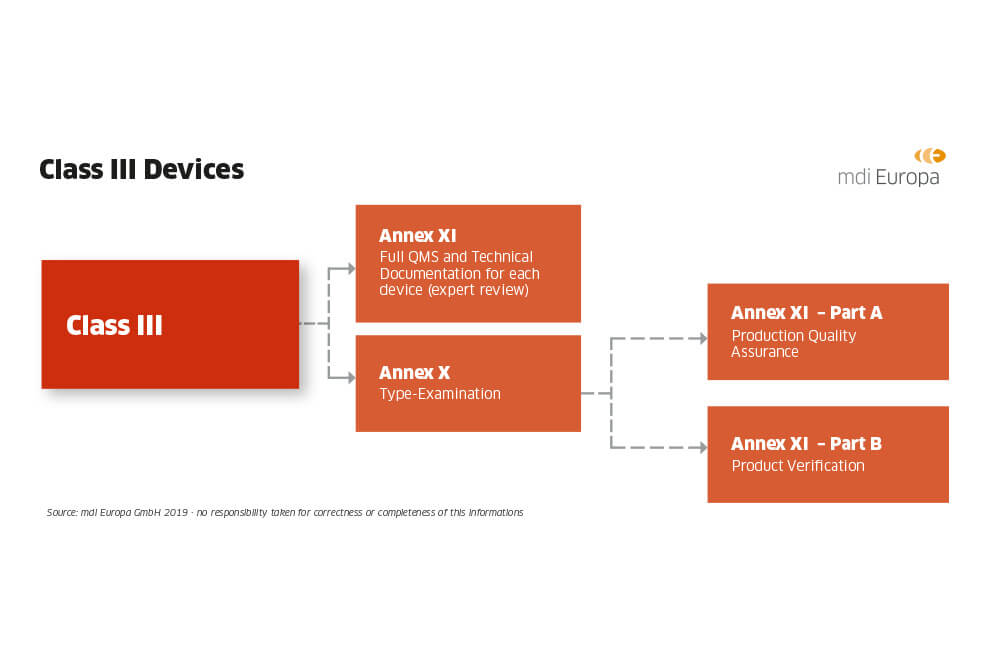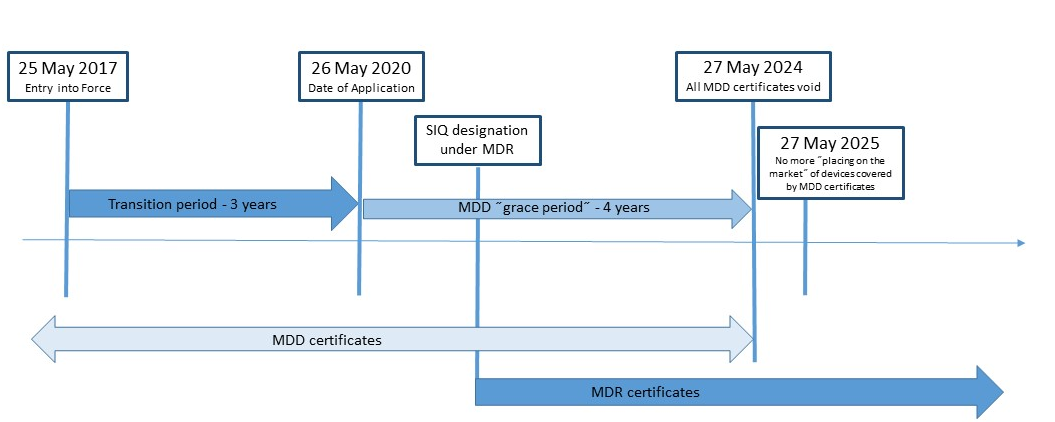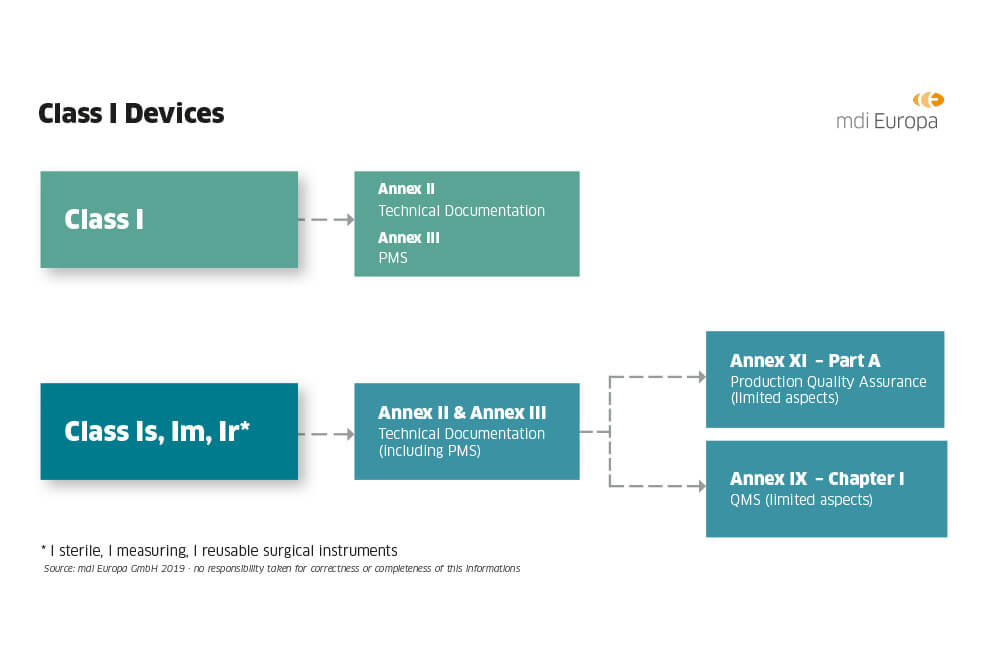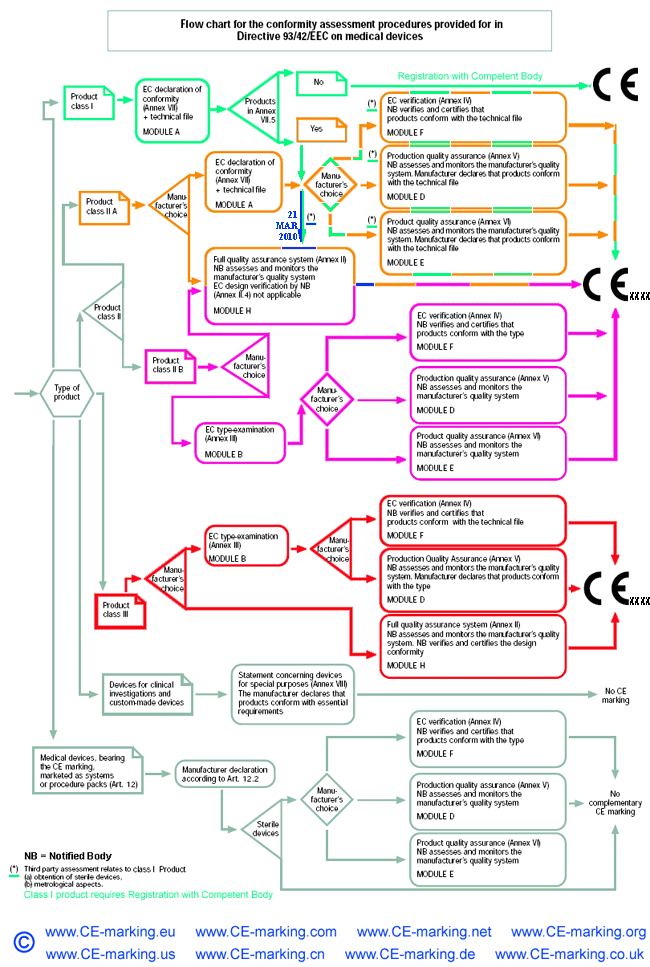
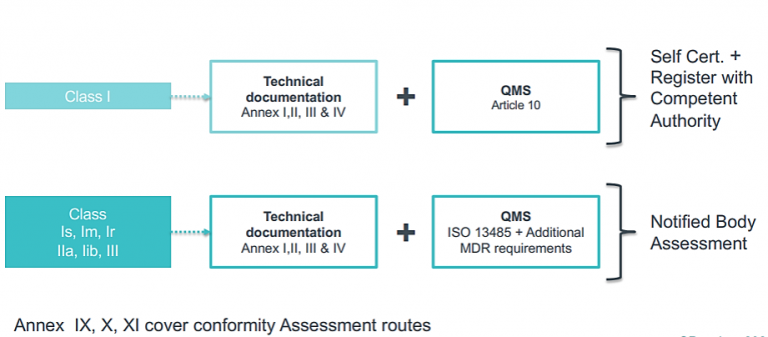
Guide on Class I (Is/Im) MDD- Medical Devices CE marking (mark) & European (EU) Authorized Representative service
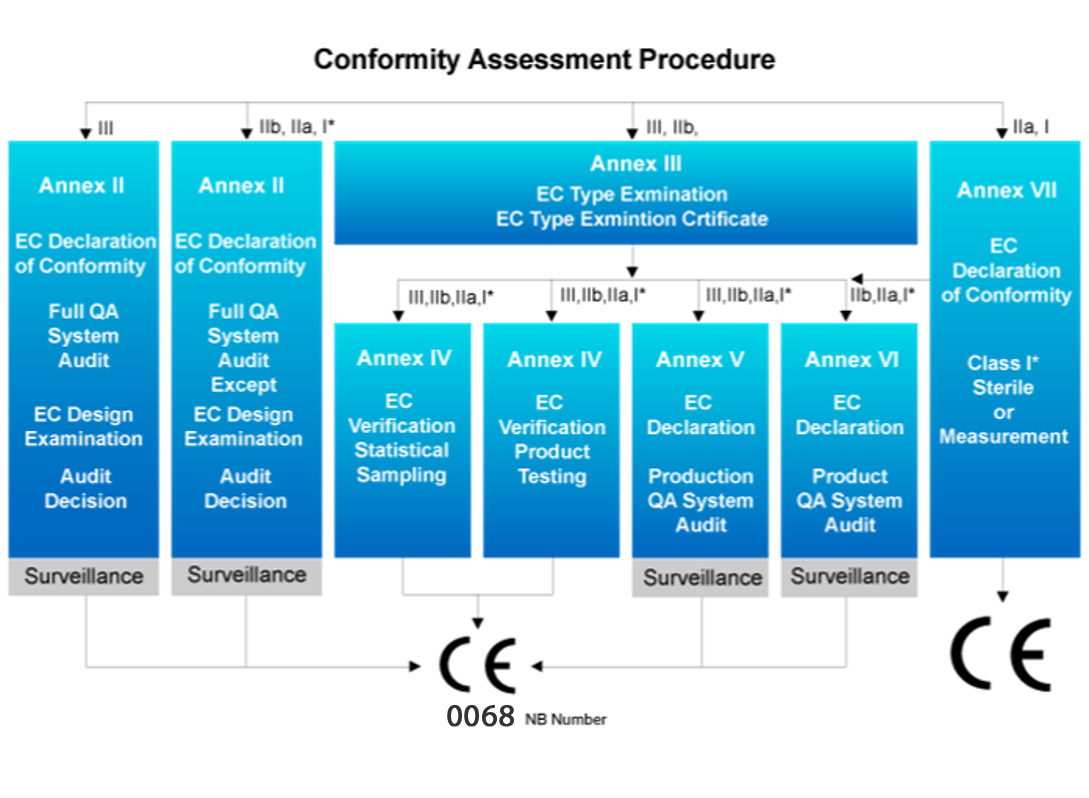
CE MDD | Global Product Certification (GPC) | Audit Auditor Training Examination Qualification Certificate Certification Body
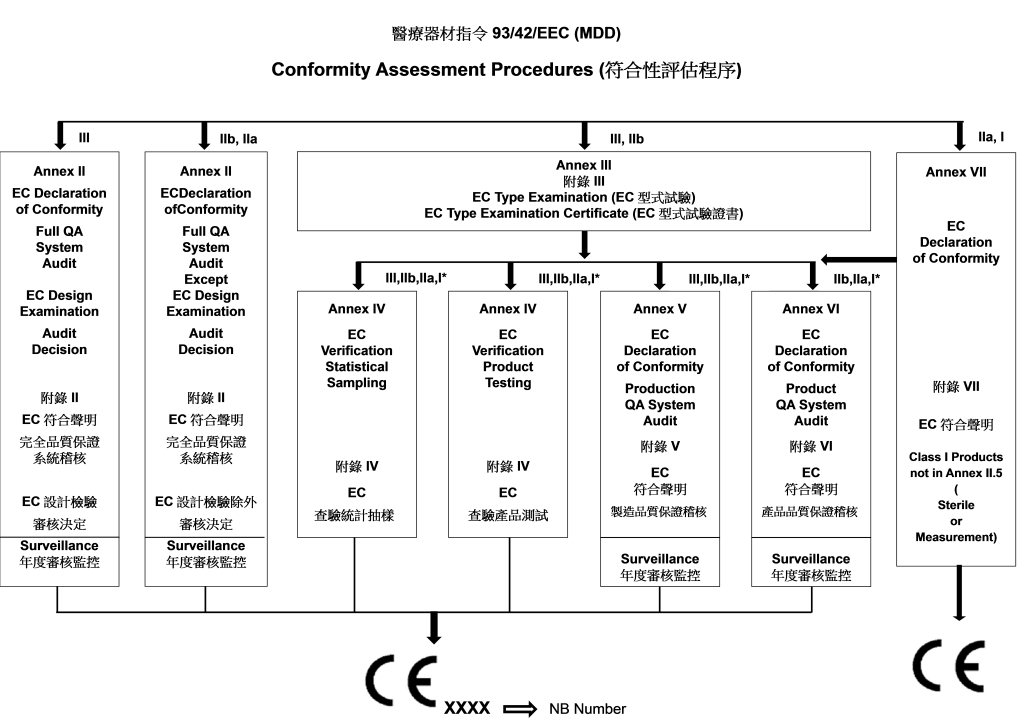
CE Marking a Medical Device under the EU MDR | Wellcome / EPSRC Centre for Interventional and Surgical Sciences - UCL – University College London

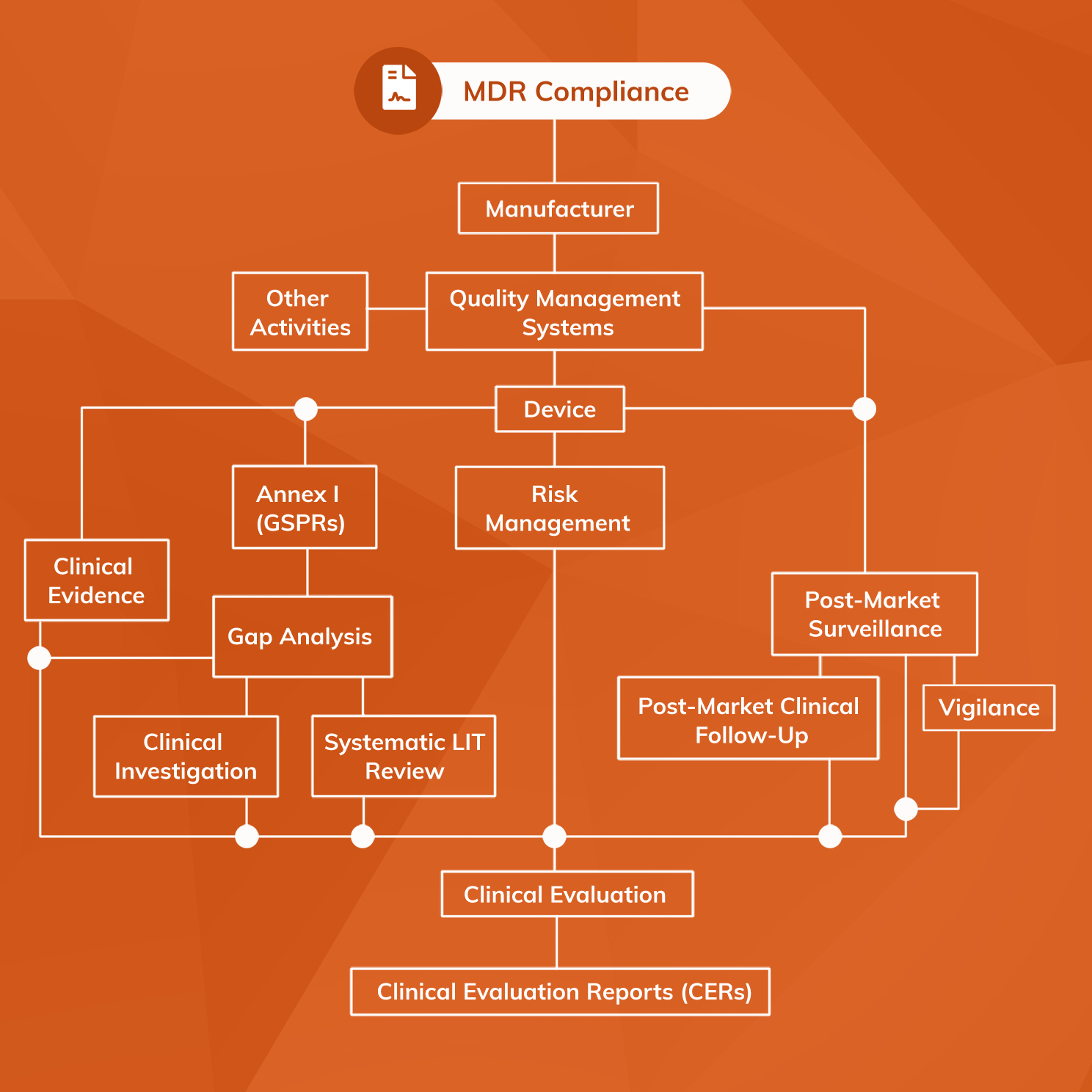


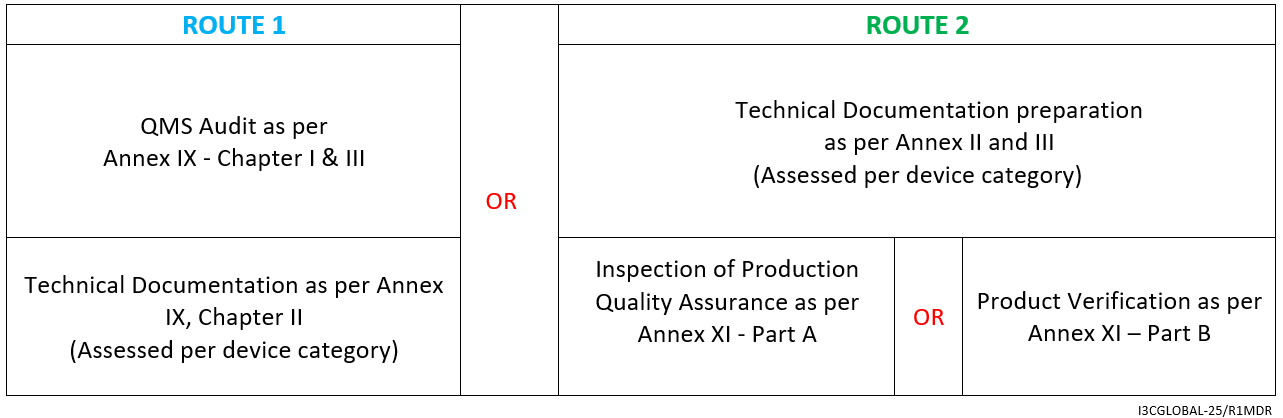


![EU MDR Update: How To Get a New Medical Device Certified? [Flow Charts] - Sofeast EU MDR Update: How To Get a New Medical Device Certified? [Flow Charts] - Sofeast](https://x6t6s6a2.rocketcdn.me/wp-content/uploads/2021/08/class-IIb-medical-devices.jpeg)