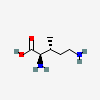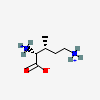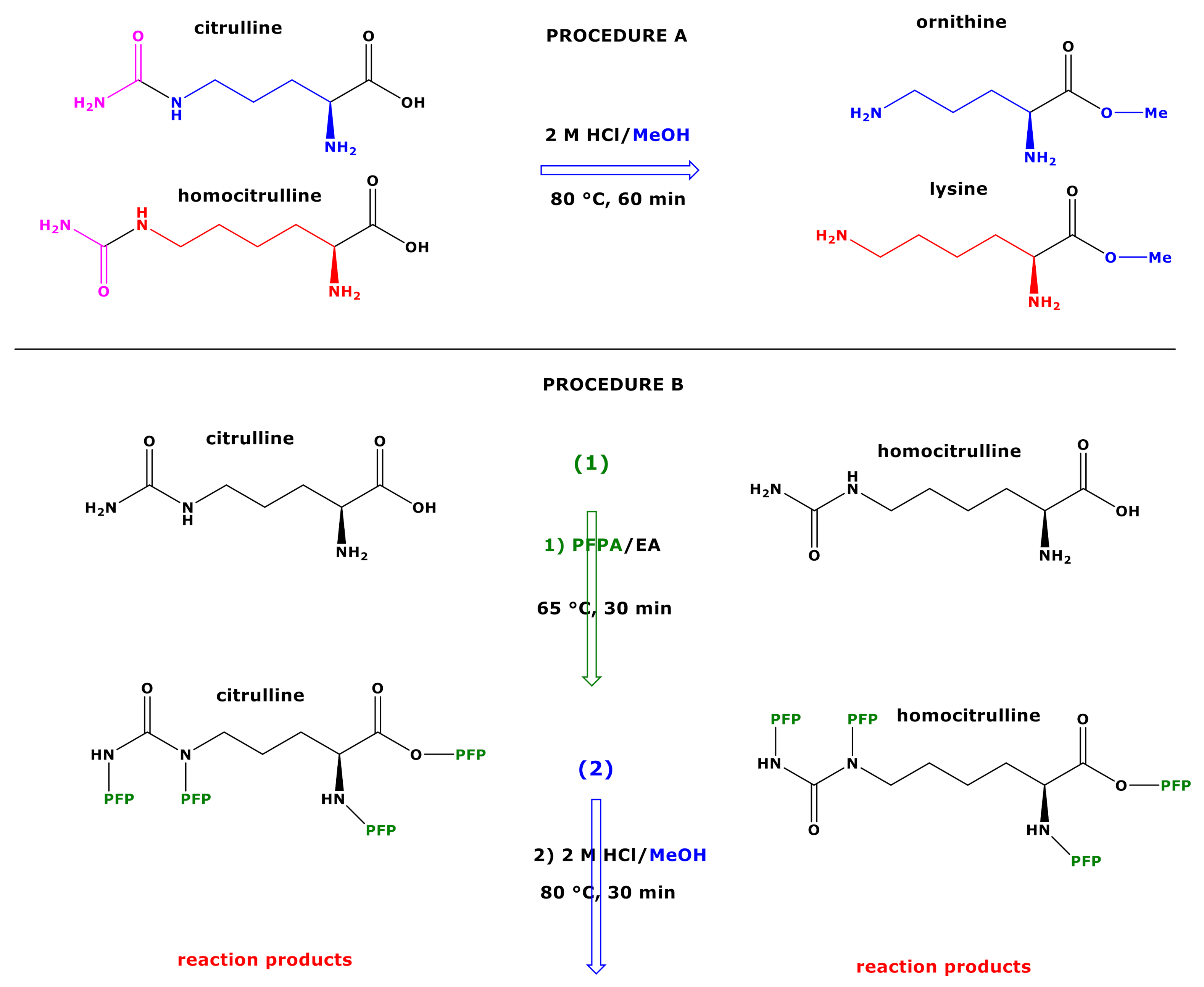
Molecules | Free Full-Text | GC-MS Discrimination of Citrulline from Ornithine and Homocitrulline from Lysine by Chemical Derivatization: Evidence of Formation of N5-Carboxy-ornithine and N6-Carboxy-lysine

Biosynthesis of the 22nd Genetically Encoded Amino Acid Pyrrolysine: Structure and Reaction Mechanism of PylC at 1.5 Å Resolution - ScienceDirect

Evaluation of Metalloendopeptidase Lys-N Protease Performance under Different Sample Handling Conditions | Journal of Proteome Research

Molecules | Free Full-Text | GC-MS Discrimination of Citrulline from Ornithine and Homocitrulline from Lysine by Chemical Derivatization: Evidence of Formation of N5-Carboxy-ornithine and N6-Carboxy-lysine

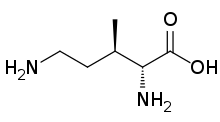
KR20120093310A - 2-amino-3-methyl-hex-5-enoic acid and its use in the production of peptides such as bacitracins - Google Patents

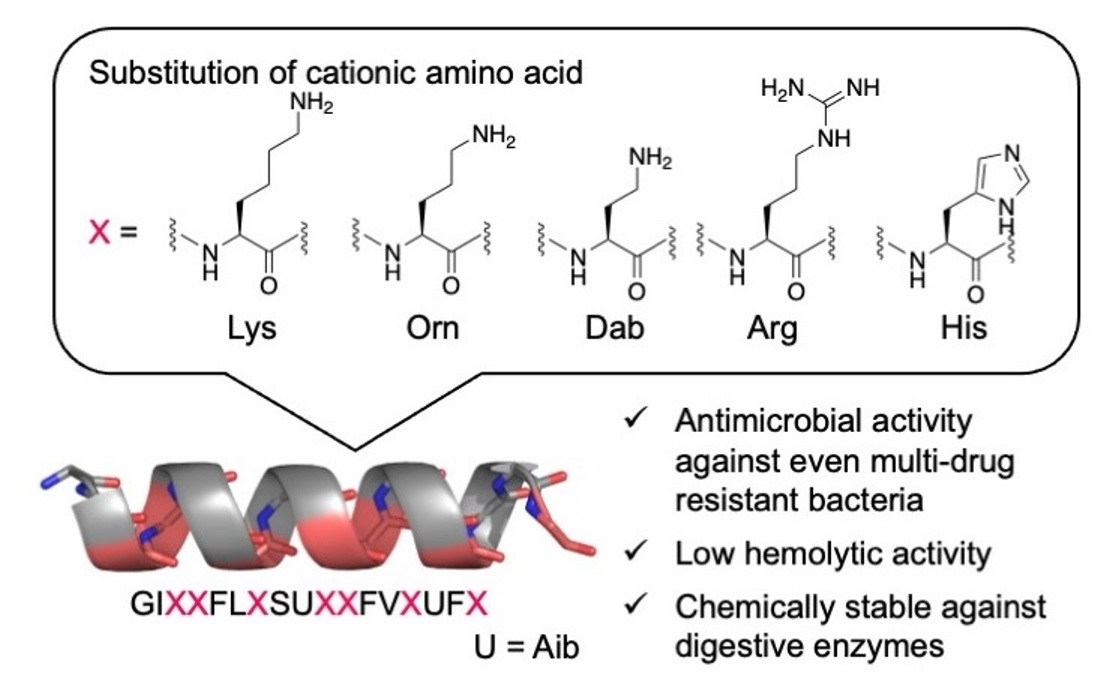
Cell-Penetrating Peptides Using Cyclic α,α-Disubstituted α-Amino Acids with Basic Functional Groups | ACS Biomaterials Science & Engineering

Structure and Reaction Mechanism of Pyrrolysine Synthase (PylD) - Quitterer - 2013 - Angewandte Chemie International Edition - Wiley Online Library

KR20120093310A - 2-amino-3-methyl-hex-5-enoic acid and its use in the production of peptides such as bacitracins - Google Patents